emc med data supply chain allows you to enter as much information about your packs as needed so that various sources such as wholesalers can access what they need.
emc supply chain acts as a single source of truth for all product information requirements that you can provide to your stakeholders. We will automatically pull through the relevant dm+d & emc information, meaning you'll only have to update the additional supply chain information.
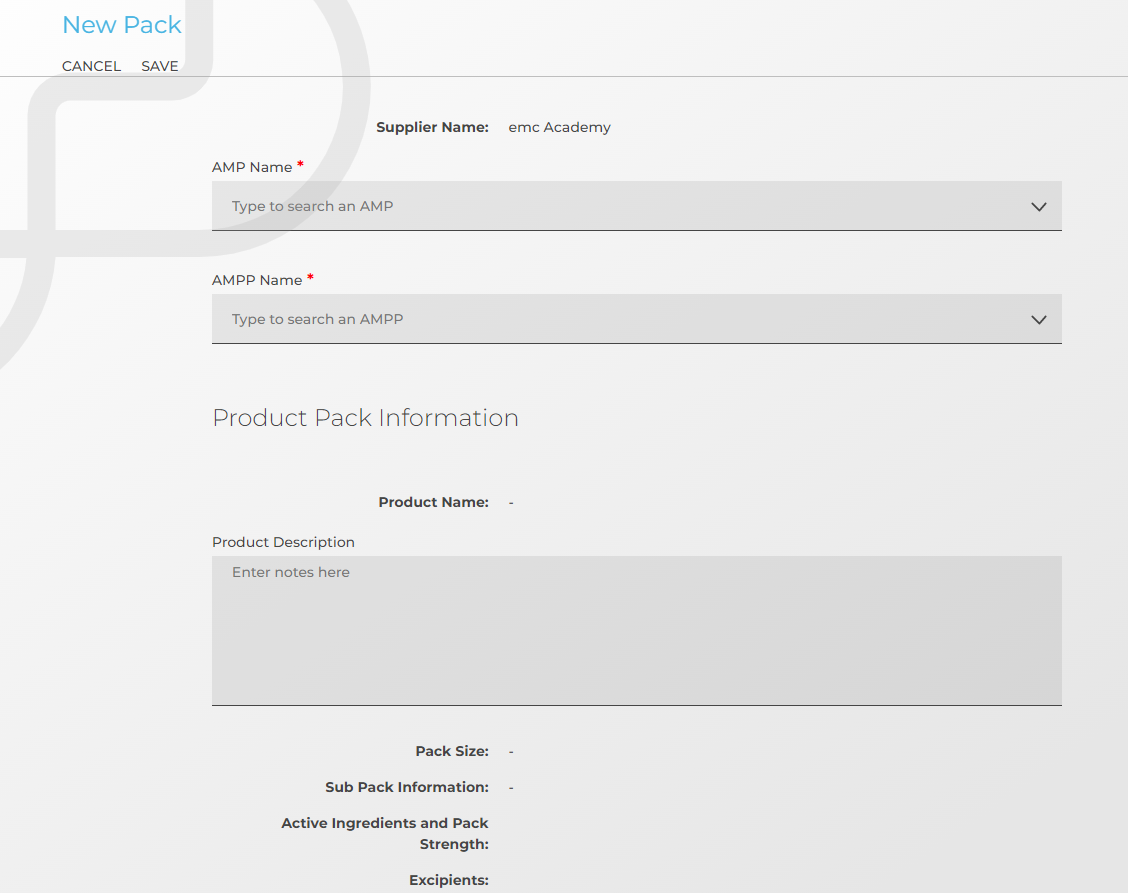
Here is a breakdown of the information that is pulled in from the dm+d, emc and also the additional information you can input:
dm+d content (Automatically pulled in)
- Supplier Name
- Product Name
- AMP Name
- AMPP Name
- Pack Size
- Sub Pack Information
- Pack Strength
- Active Ingredient
- Excipients
- Product Form
- Legal Category
- Discontinued Flag
- Product Pack Codes (ATC,BNF, GTIN)
- dm+d Price
emc content (Automatically pulled in)
- SmPC link
- PIL link
- RMM link
- Product Licence Number
- emc product ID
Supply Chain Content (To be added)
- Product Description Notes
- Flags (therapy area, ethical, generic, OTC, primary care, secondary care, cold chain, dug shortage, cytotoxic)
- Product pack images
- Product Images
- Product pack weight, height, width and depth
- Case & pallet dimensions & weights
- Quantity per outer, shipper, layer and pallet
- Storage condition & temperature notes
- Transport condition notes
- Container Restrictions notes
- PIP Code
- Internal Codes (if required)
- Shipper GTIN
- Supplier GLN
- Concession Price Flag
- Concession Price
- Concession Price Dates
The entry fields for all of this information are in a user-friendly, scrollable screen where you can go through at your own pace and enter the information as you go.
You have the option to save as a draft and come back later to add more information until you are ready to submit.
