How Do I Enter Data into emc med data (dm+d)?
Each type of submission (New Product, Price Change, etc) has required fields of data that must be completed. These required fields have been grouped into the following headings:
- Product Details,
- Effective Date,
- Ingredients,
- Excipients,
- Licence Details,
- Pack Details,
- SmPC File Upload,
- Pack Selection.
All the information required for each type of submission is displayed on the same page. Fields that are marked with a red asterisk (*) indicate a mandatory field.

When clicking on a section to enter information, additional text will appear providing guidance on what is required.
If you cannot complete the submission (e.g. if another user needs to provide information) you can save the submission as a draft. Any registered emc med data dm+d Data Manager can view and add information to a submission.
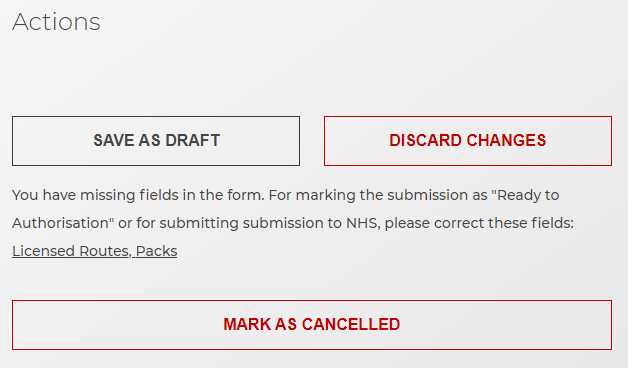
You will not be able to submit a document that has required fields missing.
If there are required fields missing, the sections with missing details will be highlighted.
You also have the option to discard changes made or mark the submission as cancelled.
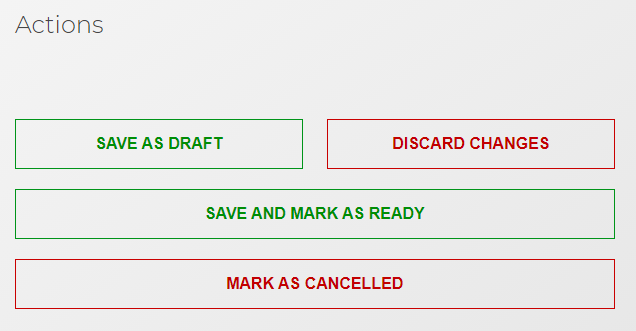
On successfully completing all fields you are able to progress the submission to the next stage. The status depends on the permission level the user has for emc in-demand:
Level 1 permission - allows users to create, view, and edit submissions but not authorise for submission to the NHS BSA. Once a submission is ready, selecting 'save and mark as ready' will update the submission status to 'ready for authorisation'.
Level 2 permission - allows users to create, view, and edit submissions as well as authorise them for submission to the NHS BSA.

![HIRES_DP220702 emc academy 2022 logo WO1.jpg]](https://support.datapharm.com/hs-fs/hubfs/HIRES_DP220702%20emc%20academy%202022%20logo%20WO1.jpg?height=50&name=HIRES_DP220702%20emc%20academy%202022%20logo%20WO1.jpg)