What is an RMM?
A risk management plan may require the dissemination of educational materials – these are known as Educational Risk Management Material’s or RMMs
These additional educational materials may be published on the emc alongside their respective SPCs and PILs. All RMMs published on the emc must have been approved by the MHRA
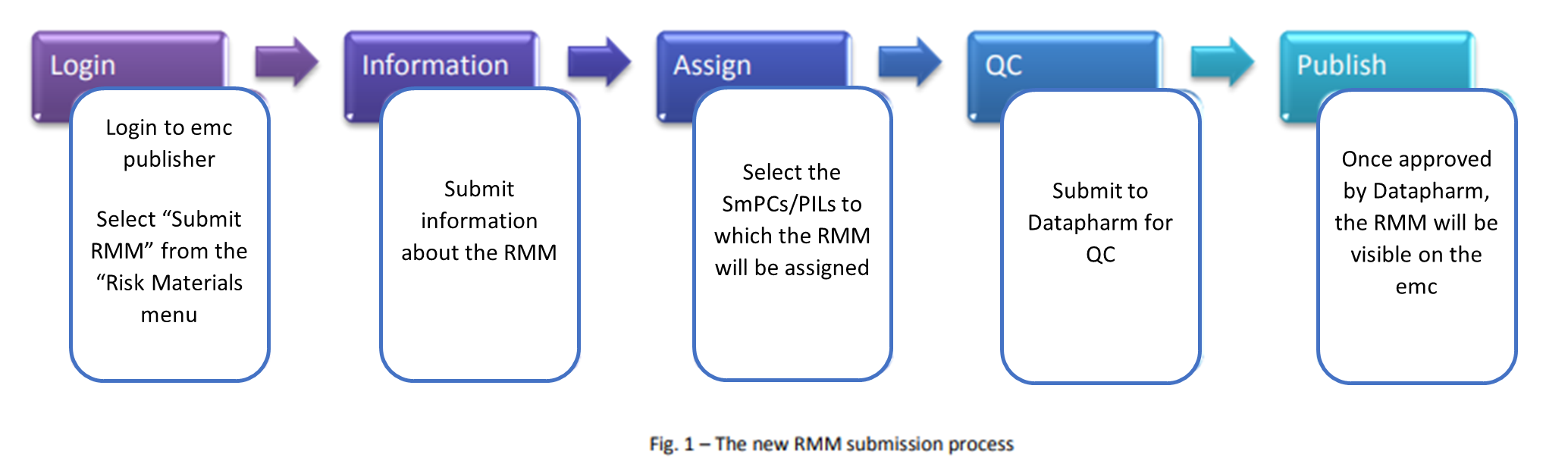
Submitting a new RMM using emc publisher
Once you have logged in, use the easy-to-follow wizard to submit your RMM:
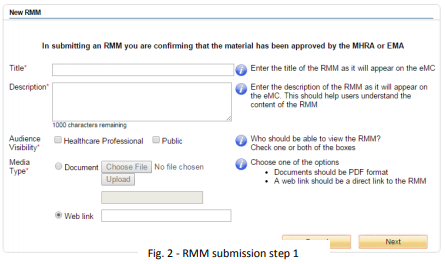
The first screen of the wizard requires you to enter various details about the RMM. See Appendix 1 (below) for help with the fields that will need to be completed during submission.
You can submit a web link by adding the URL in the web link box
To upload an RMM in PDF format:
1. Select ‘Document’ as the media type
2. Click on ‘Choose File’ and select the PDF from your local storage
3. The file name will appear next to the ‘Choose File’ button
4. Click ‘Upload’
5. The PDF file name will appear in the box below the ‘Upload’ button if
the file has been successfully uploaded.
Assign documents to the RMM
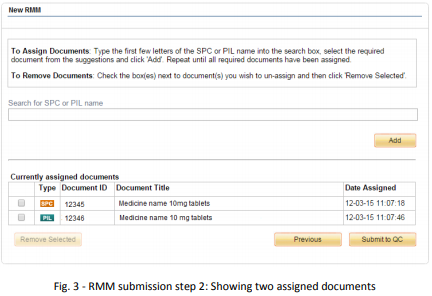
The second stage of the submission process allows you to assign documents to the RMM. This determines which of your emc SmPCs and PILs pages the RMM will be viewable from.
To assign SmPCs or PILs:
1. Type the first few letters of the SmPC or PIL name
2. Select from the list of suggestions
3. Click ‘Add’
One or more SmPCs or PILs can be assigned to an RMM. When at least one SmPC or PIL has been assigned the ‘Submit to QC’ button will become active. Once you have assigned all the relevant SmPCs and PILs to the RMM, click this button to pass the RMM to Datapharm for quality control
The QC process
All RMMs are subject to quality control checks before publication on the emc
When you submit your RMM for QC, the Datapharm quality control team will be notified. This process is usually completed within one working day of submission
Please Note 📝
If the RMM does not meet the QC criteria it will be given a status of ‘needs attention’. The submitter will be sent an email informing them that the RMM has failed the QC process and should be amended before being re-submitted for QC.
Publishing process
If the RMM meets the required criteria then it will pass the QC process and will be displayed on the emc website as a separate tab on the product page. No additional publishing stage is required so you should not submit the RMM unless it is ready for display on the emc
Updating an existing RMM
An RMM can be updated for a number of reasons:
- To change the title or description
- To update the media type (PDF document or web link)
- To change the SmPCs/PILs it is assigned to
These changes can be made to amend an RMM that has failed the QC process or to update an RMM that has previously been published on emc
To update an existing RMM follow these simple steps:
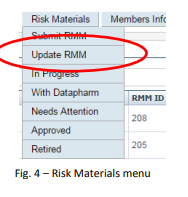
1. Click the "Risk Materials" button to display the menu
2. Click on ‘Update RMM’ (see Fig.4)
3. Select the RMM you wish to update from the drop-down list
4. Click on ‘Next’
5. You are now in the ‘Update RMM’ wizard which is similar to submitting a new RMM (see ‘Submitting an RMM’)
Once you have updated the RMM, and/or changed the documents to which it is assigned, it will need to be passed to Datapharm’s QC team. It will be subject to the same checks as a newly submitted RMM (see ‘The QC Process’ above).
Please Note 📝
1. If an RMM has been previously approved it will remain available on the emc and will only be replaced once the updated version has passed the QC process.
2. If you choose to update a retired RMM, it will become visible again on the emc once the updated version has passed the QC process.
Retiring an RMM
An RMM that is currently available on the emc can be retired. Once it has been retired, it will no longer be visible on the SPC/PIL pages to which it had been assigned. To access the ‘retire’ function follow these simple steps
1. Click the button in the header to list all of your RMMs
2. Locate the RMM you wish to update from the list, or use the search function on the left of the screen to narrow down the number of RMMs listed
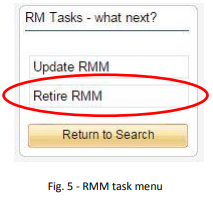
3. Click on the title of the RMM you wish to retire, this will display the RMM details and present a new menu on the left of the screen (See Fig. 5)
4. Click ‘Retire RMM’
5. Confirm that you wish to retire the selected RMM
Audit and Tracking
As with SmPC and PIL submissions, emc publisher provides tracking information for the current version of the RMM. A history of the versions of the RMM is also maintained. Both of these functions can be accessed from the RMM details page.
Appendix 1
Title - This is the name of the RMM as it will appear on emc. The name should give a clear indication of what the RMM is and, if applicable, the target audience e.g. Medicine name Patient Booklet
Description - This is the description of the RMM which will appear underneath the title. It should help users understand the content of the RMM and how it will benefit them.
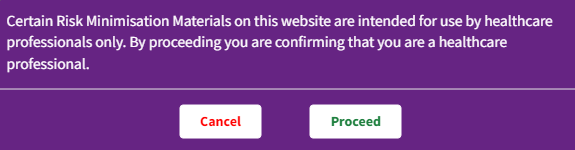
Audience visibility - This sets the intended audience of the RMM. Choose either healthcare professional or public, or both. If this is set to healthcare professional, the user will be required to self-certify that they are a healthcare professional before they can access the material.
Media type - The RMM should be either a document, in PDF format, or a link to a website hosting the RMM. A size limit applies to uploaded documents; therefore submitted PDFs should be under 5MB in size. A web link should be a direct link to the RMM or web page containing the RMM.