When updating a PIL, emc publisher has the functionality in place which will allow users to 'pre approve' the publication of the PIL.
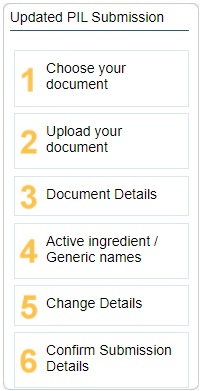
When preapproving the PIL, you will first go through the 6 submission steps where you upload the new document file and alter any details that may have changed for the latest version (https://support.datapharm.com/knowledge/what-is-dms-and-process-overview)
Once you have confirmed those submission details in section 6, you will then be led to the below screen where you can review and publish/pre approve your submission.
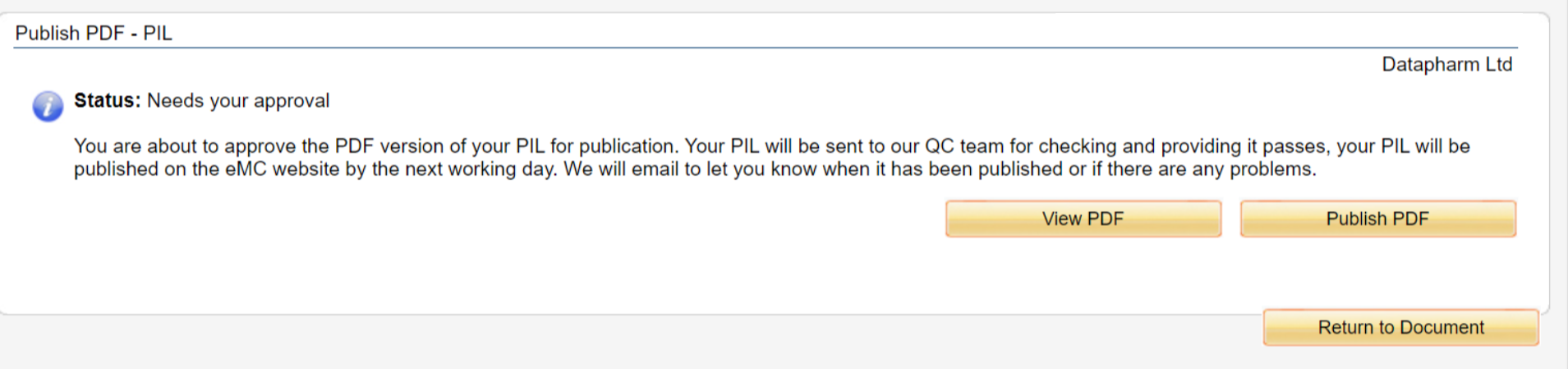
View PDF - This would be for users to have a final check of their uploaded document before completing the submission.
Publish PDF - This would complete your submission and pass onto Datapharm's QC team who would go through one final QC process before publishing onto the emc website within 1 working day.
Please be advised 📢
PIL and RMM documents are pre-approved at the point of submission, so once they have passed our QC process, they will go live on the emc website.
SmPC documents are NOT pre-approved. The original submitter will receive an email notification when their document has passed QC and they can then log in to emc publisher to make the final "approve and publish".
